The Rare Diseases Act of 2002 — Part 2 of 4
How Rare Disease Became Federal Infrastructure
Part 1 examined how the Orphan Drug Act of 1983 used incentives to correct a market failure—and why it worked. But that law was never intended to create a permanent system.
In 2002, that changed. With the Rare Diseases Act, rare diseases stopped being an exception to the medical marketplace and became embedded in the federal research infrastructure itself. This essay examines how a temporary solution quietly sowed the seeds for something much larger, with consequences that were not anticipated at the time.
The Orphan Drug Act of 1983 has proven its value. By offering tax credits, grants, and seven years of market exclusivity, it spurred hundreds of orphan drug designations and approvals where none had existed before. What was once a neglected corner of medicine began to attract real investment. But the law’s incentives were narrowly focused on drug development—temporary bridges over a market gap. Rare diseases were addressed as exceptions, not yet embedded as a standing priority within the medical system.
In 2002, Congress took the next step. On November 6, President George W. Bush signed the Rare Diseases Act into law. Unlike the Orphan Drug Act, which relied on pharmaceutical incentives, this legislation embedded rare diseases directly into the federal research infrastructure at the National Institutes of Health (NIH).
The Act’s congressional findings captured the scale of the problem at the time: more than 6,000 known rare diseases affecting an estimated 25 million Americans, many of whom lacked effective treatments due to limited commercial interest. The law sought to address this by formalizing rare disease research through several key mechanisms:
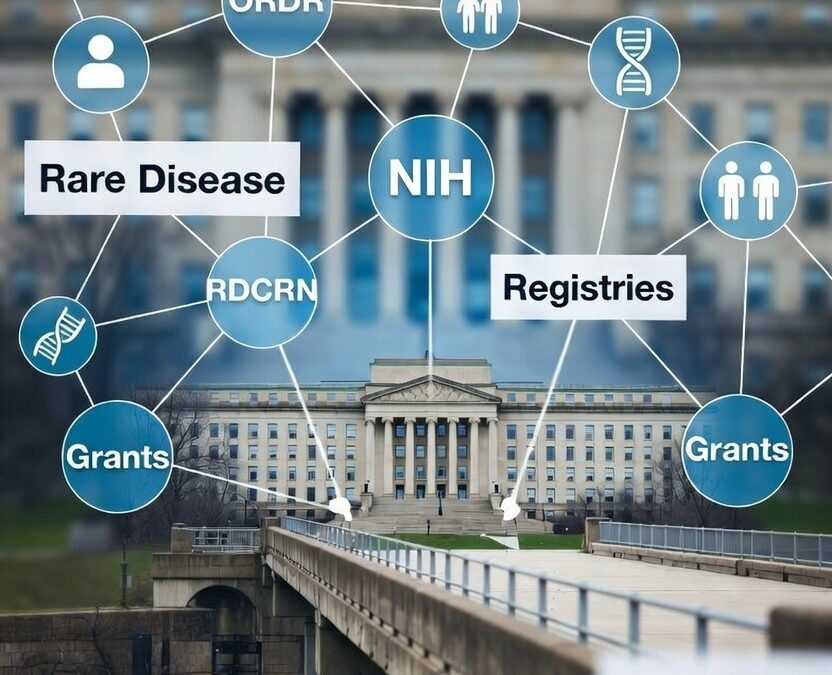
- Establishing a statutory Office of Rare Diseases—now the Office of Rare Diseases Research (ORDR) within the National Center for Advancing Translational Sciences—to coordinate NIH efforts
- Recommending a national research agenda for rare diseases
- Supporting education, workshops, and symposia to identify research opportunities
- Authorizing grants for regional centers of excellence, which led to the creation of the Rare Diseases Clinical Research Network (RDCRN) to support collaborative clinical studies, training, and patient engagement
The Act also standardized the definition of a rare disease as any condition affecting fewer than 200,000 people in the United States—the same threshold used in the Orphan Drug Act—and authorized modest appropriations (approximately $4 million annually from FY 2003–2006). Though limited in dollar terms, this funding signaled a long-term federal commitment.
The shift was profound. Rare diseases were no longer addressed solely through targeted incentives; they became a formalized category within federal medicine, complete with dedicated offices, recurring grant pipelines, disease registries, and collaborative frameworks linking government, academia, industry, and advocacy organizations. Across this ecosystem, sustained participation—not resolution—was rewarded.
Since 2002, this infrastructure has driven substantial investment. Federal funding coordinated through ORDR, RDCRN cycles, and related NIH grants is estimated at $2–4 billion, with RDCRN alone exceeding $500–600 million across its five funding cycles. The system expanded research capacity, built networks, and increased visibility for rare conditions. But the numbers also reveal a deeper paradox. While the 2002 Act referenced roughly 6,000 known rare diseases, the recognized total has since climbed well beyond 10,000. This increase is often attributed to advances in genetics, molecular diagnostics, and data aggregation—suggesting that many conditions always existed but were previously unidentified. Yet that explanation is incomplete.
Over the same period, Americans have experienced unprecedented and cumulative exposure to thousands of synthetic chemicals, food additives, pharmaceuticals, and environmental contaminants—many approved under fragmented regulatory oversight and rarely studied for long-term, low-dose, or synergistic effects. Rather than asking whether this expanding chemical burden is contributing to disease emergence, the system has largely focused on classification and management after the fact. More diseases are cataloged, more subtypes defined, and more patients enrolled—not necessarily because detection has improved alone, but because upstream causes remain unaddressed.
Rare disease was now a permanent federal program to manage, not a temporary gap to close. No built-in mechanisms required measuring reductions in disease prevalence, cures achieved, or fewer patients needing lifelong care. Success became increasingly tied to activity—more studies, more centers, more funding—rather than to resolution.
What began as compassion had evolved into something enduring: a formal system with billions invested, expanding infrastructure, and a growing catalog of recognized diseases. After more than four decades, what has that investment actually produced?

Linda Wulf
Linda Wulf is a cancer rebel, advocate, and independent researcher. Diagnosed in 2023 with primary CNS lymphoma, she declined standard chemotherapy and pursued a root-cause, immune-supporting path. Twenty-three months cancer-free via root-cause approach.