by Linda Wulf | Oct 4, 2025 | Main Blog |
A Broken Promise of Food and Drug Safety
Most Americans believe the FDA exists to keep them safe. The reality is stark: over 7,000 unique chemicals circulate in our food, packaging, drugs, and personal care products, and the majority have never been meaningfully reviewed for long-term safety.(1)

Instead of acting as a watchdog, the FDA functions as a filing cabinet for industry submissions, relying on the ‘Generally Recognized as Safe (GRAS)’ loophole. Companies can self-certify their own ingredients as safe, bypassing independent oversight. Of nearly 4,000 substances in the FDA’s food database, about 3,670 were never actively reviewed by the agency.
The Cost of Industry Capture
Lobbying has tilted the playing field. Since 1998, food and pharma interests have spent nearly $8 billion lobbying against stronger oversight. Efforts to close the GRAS loophole or restrict hazardous substances have been blocked for decades. Even the EPA’s endocrine disruptor program, launched in 2009, has not removed a single chemical from U.S. products.
This isn’t about a few questionable additives — it is systemic. Americans are routinely exposed to thousands of inadequately tested substances across food, packaging, drugs, and cosmetics. (2)
Case Study: A Cancer Rebel’s Perspective
They told me I had “an inoperable, incurable brain cancer.” But ‘incurable’ didn’t mean there was no cause — it meant the guidelines didn’t have a drug for it.” In Medical Guidelines: A Cancer Rebel’s Perspective (3), I describe how standard medical guidelines reduced my disease to a genetic fluke and a drug protocol. The root causes — years of unresolved dental infections and chronic inflammation — were dismissed with the words: “We don’t do teeth.”
This reflects the drug-first trap. Guidelines are written by panels intertwined with industry, incentivized to define “cure” as a prescription rather than prevention. Patients are steered toward toxic treatments and away from investigating underlying infections, environmental exposures, or chemical triggers.
My story is not unique. It is a lived example of how the FDA’s toxic web and the healthcare system’s perverse incentives combine to ignore causes, enforce compliance, and profit from endless treatment.
A Healthcare System That Ignores Root Causes
The problem doesn’t stop with chemicals. In medicine, ‘cure’ is defined almost exclusively as a drug. Since the Orphan Drug Act (1983), billions have flowed into drug pipelines, while root causes like chronic infections, inflammation, endocrine disruptors, and diet-driven dysfunction are dismissed as irrelevant.
Insurance and provider lobbying reinforce this drug-first system. Insurers decide what gets covered — often excluding critical tests that could reveal root causes — while providers are incentivized to treat symptoms, not prevent disease. Patients seeking answers about environmental or infectious causes are too often ignored or told “that’s not covered.”
The System Is Working—For Them
This isn’t a broken system. It’s a system working exactly as designed:
– For industry: endless new drugs, market exclusivity, and record profits.
– For regulators and policymakers: fees, royalties, patents, and revolving-door jobs.
– For insurers and providers: coverage restrictions that block root-cause testing, feeding patients back into the drug/procedure pipeline.
– For the public: exposure to thousands of inadequately reviewed chemicals, resulting in chronic health issues — obesity, infertility, diabetes, cancer — while being left uninformed about the true risks.
What Real Reform Looks Like
To protect public health, the FDA and policymakers must:
1. End the GRAS self-affirmation loophole — require independent FDA review of all additives.
2. Consolidate oversight — unify food, drug, and cosmetic regulation under a single accountable body.
3. Restrict lobbying influence across the entire healthcare system — not just food and pharma, but also insurers and provider networks whose lobbying skews coverage decisions and testing.
4. Ban financial conflicts of interest — policymakers and regulators cannot participate in profits, royalties, or patents tied to the industries they oversee.
5. Build a transparent public database of all chemicals in food, drugs, and cosmetics with plain-language safety summaries.
6. Re-center healthcare on root causes — covering and encouraging tests that detect infections, inflammation, endocrine disruption, and environmental exposures rather than defaulting to drugs.
Until Then
Americans must act as their own watchdogs — reading labels, scanning products with independent tools like Yuka, and demanding accountability. They need to be fully informed and must make the choice to stop consuming the poisons that regulators have failed to remove from the market.
(1) THE TOXIC WEB
(2) NO CAUSE NO CURE
(3) NAVIGATING MEDICAL GUIDELINES – A CANCER REBELS PERSPECTIVE
by Linda Wulf | Sep 8, 2025 | Main Blog |
Medical Guidelines
When I was diagnosed with primary central nervous system lymphoma (PCNSL) in November 2023, my doctors called it a “rare disease.” The young doctor breaking the news nearly wept as she told me my diagnosis and encouraged me with “DO NOT GOOGLE” this. When I googled it, I could see why—it was horrible! Best case scenario was a pretty short stint left on this earth. Some do better than others, the words were – incurable, but treatable.
The preferred path recommended for me was clear: high-dose chemotherapy, rituximab infusions, temozolomide, and eventually autologous stem cell transplant (ASCT). And if that failed, maybe, just maybe I would be lucky and get into a clinical trial for a new immunotherapy drug when the cancer recurred. And I was assured it would return – AGGRESSIVELY if I didn’t stick with the regimen.
Always a bit of a rebel at heart, I chose a different path.
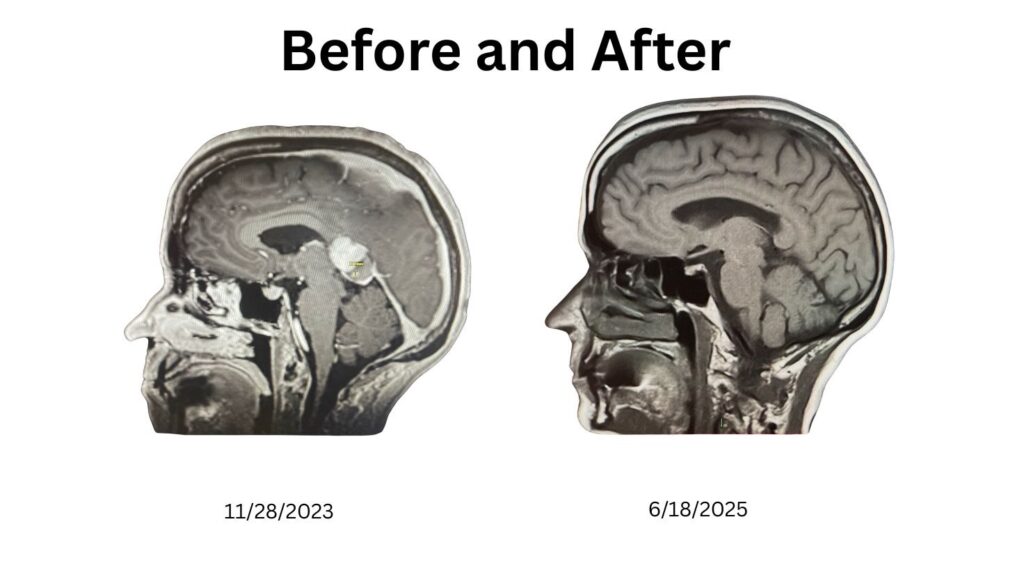
Rather than becoming the kind of “warrior” who fights my own body with toxic regimens, I chose to be a cancer rebel. I leaned into breathwork, fasting, whole foods, meditation, exercise and chemical-free living. Along the way, I eradicated a chronic dental infection—a root cause I could clearly trace to my illness. Within months, my scans showed no active disease. These are my scans, the left taken at the Mayo Clinic – the right at Reno Diagnostics. Different technology. (Note: I may not have pulled the exact same image from the CD. I am not a radiologist, so if an expert would like to compare my scans, please reach out.)
The Limits of Guidelines
The NCCN Guidelines for PCNSL describe my cancer as the result of genetic mutations in lymphocytes, with risk factors like HIV, Epstein-Barr virus, autoimmune disease, organ transplants, or simply being old. Other than being 64, none of these applied to me. There was no mention of dental infections, environmental toxins, or other possible root causes.

As mentioned earlier, the treatments they recommended for me—methotrexate, rituximab, temozolomide, and eventually ASCT—are intense and risky. Nothing in the guidelines suggests they have any real understanding of how the cancer developed or how the body itself might help or participate in the healing process. Cure is described as rare, and clinical trials are recommended for everyone.
Not Just My Cancer
This drug-centered playbook is not unique to PCNSL. Consider prostate cancer, one of the most common cancers in men. The NCCN Guidelines for Prostate Cancer emphasize surgery, radiation, and hormone therapy, but still highlight clinical trials, recurrence management, and long-term drug dependence.
They frame the cause as “genetic changes” or “just aging.” Lifestyle is brushed aside. And while early-stage prostate cancer is labeled “often curable,” advanced cases shift right back to the same language of management, remission, and control.
Here’s the comparison in black and white:
| | |
| High-dose chemo, rituximab, radiation, stem cell rescue | Surgery, radiation, hormone therapy, active surveillance, chemo for advanced |
| Explicitly recommended for every patient; framed as a top option | Mentioned as options; pitched as cutting-edge research |
The details differ, but the pattern is identical:
- Narrow causes (genetics, age).
- Aggressive drug/surgery protocols.
- Clinical trials positioned as the “next hope.”
- Pharma funding quietly underwriting the guidelines.
Where Patients Fit
In neither guideline set do patients find advice on self-care, diet, detox, or root cause exploration. Instead, the role assigned is passive: accept treatment, manage side effects, stay in line. The message is clear—control is not in your hands.
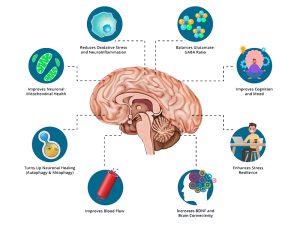
But my story, and the stories of others, show otherwise. Addressing infection, nutrition, breath, and toxins gave my body the space it needed to heal. Along the way, I learned about neurogenesis—the brain’s ability to grow new cells—and I wrote about it in “The Brain’s Amazing Ability to Regenerate: Neurogenesis & My Journey.”
That perspective—that you are not powerless—is missing from official playbooks.
Rebel, Don’t Just Comply
The NCCN guidelines are one perspective, not the only path. They are influenced by drug companies, written by panels of doctors tied to the very industry that profits from perpetual treatment.
Take what’s useful from them—but question the rest. Explore your own root causes, and above all, examine the substances you are consuming.
Look at your body not just as a battlefield but as a system capable of healing if supported. If you’d like to see the specific protocols I applied, I detailed them in “Breathing New Life into Cancer Recovery: A Data Backed Challenge to Conventional Protocols.” I’m living proof that you can rebel against the system and still find hope, healing, and clear scans.
Lastly, we need to recognize how our own governance systems are keeping us sick. I explored that in “The Toxic Web – Inadequate Governance, Overlapping Databases & Big Money Keeping America Sick.”

by Linda Wulf | Sep 1, 2025 | Main Blog |
Billions flow into drug pipelines while root causes like infection, inflammation, and chemicals are ignored.
They told me I had an ‘incurable, inoperable brain cancer.’ But the word incurable wasn’t true — it was a reflection of how our system defines cure. In medicine today, cures almost always mean drugs. And when the cause doesn’t fit that drug-first narrative, it gets ignored.
My cancer didn’t come from bad luck or a random mutation. It followed years of chronic dental infections — a connection my doctors dismissed with the words: *’We don’t do teeth.’* That single line summed up the blind spot baked into our healthcare system: it doesn’t look for causes, it looks for prescriptions.

The Incentives That Shape Medicine
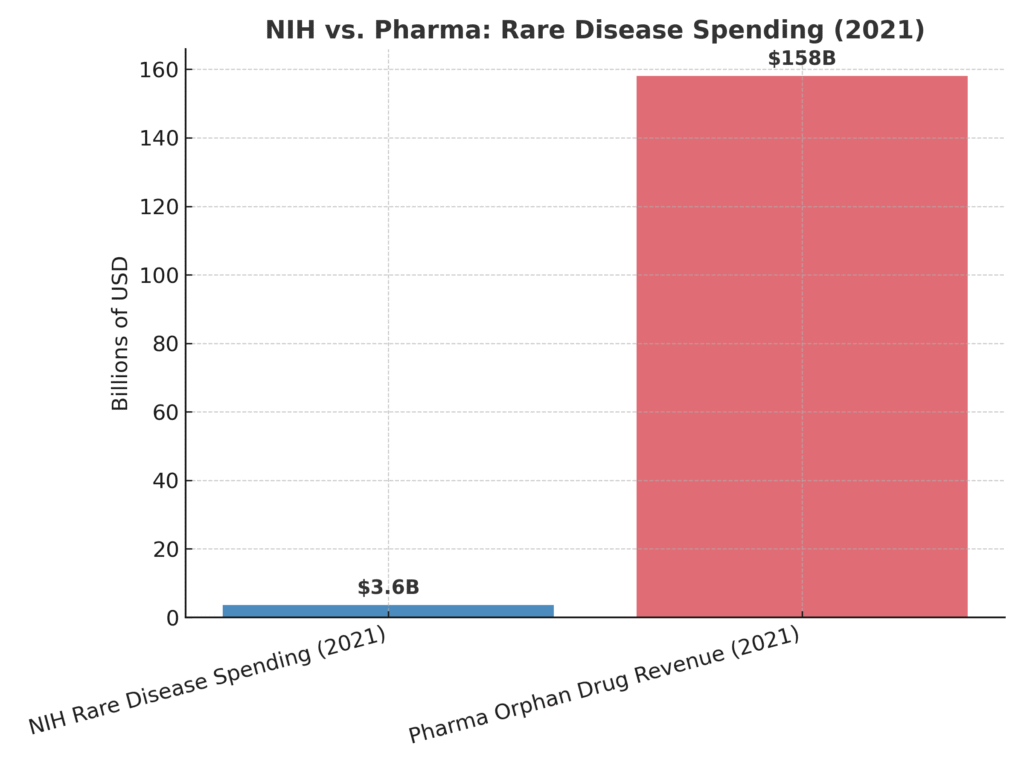
Since the Orphan Drug Act of 1983, a ‘rare disease’ has been defined as one that affects fewer than 200,000 Americans. The law created powerful incentives for companies: seven years of market exclusivity, tax credits for clinical trials, and waived FDA fees. In 2002, the Rare Diseases Act expanded NIH funding, creating the Office of Rare Diseases Research. Both laws were designed to stimulate innovation — and they did. But the innovation was channeled almost exclusively toward drugs, not prevention, not causes. Billions have flowed into the pipeline.
The government doesn’t just regulate this system — it participates, benefiting from patents, royalties, and partnerships. Meanwhile, the pharmaceutical industry reaps trillions in revenues. The balance is obvious: cures are defined as drugs, not as eliminating the conditions that cause disease in the first place.
A Timeline of Oversight—and Abandonment
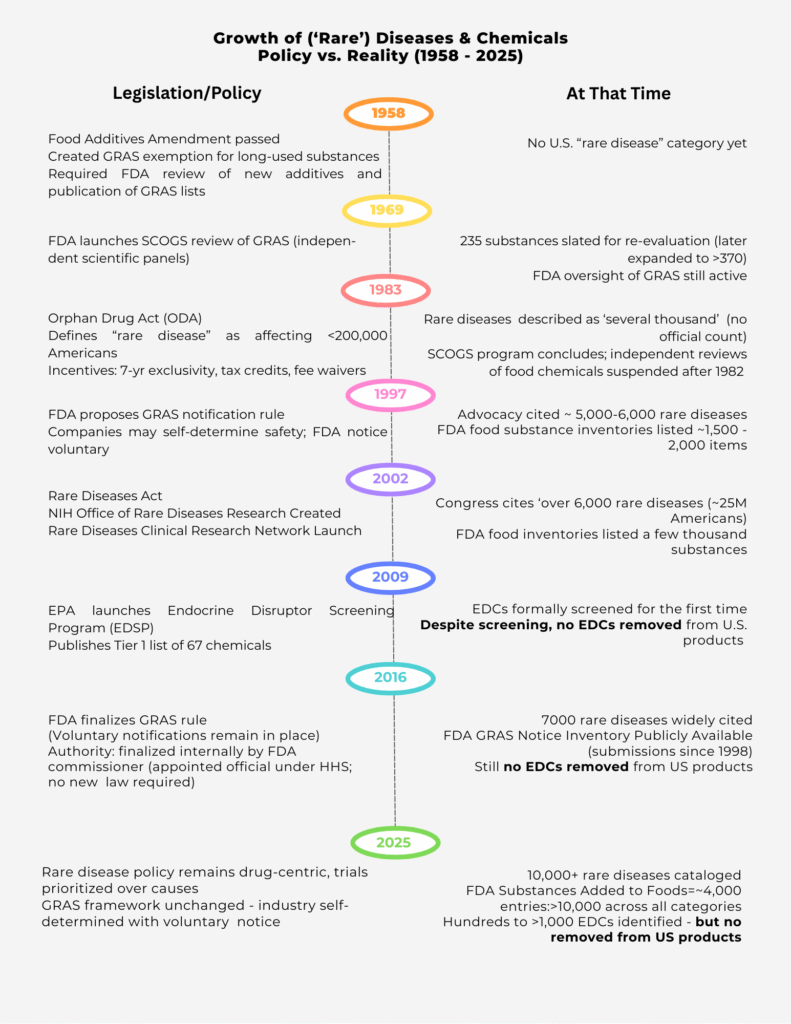
The government once had a more balanced approach. In 1958, the Food Additives Amendment created the ‘generally recognized as safe’ (GRAS) category. By 1969, FDA launched the SCOGS program, where independent scientific panels reviewed hundreds of food chemicals. Those reviews concluded in 1982, and no significant removals followed. After that, independent oversight was suspended. In 1983, the Orphan Drug Act shifted the focus squarely toward drugs.
In 1997, FDA proposed a new rule allowing companies to self-certify chemical safety with voluntary notification to the agency. By 2016, the GRAS rule was finalized — not by Congress, but internally by the FDA Commissioner, an appointed official. That single decision cemented a framework where industry now polices itself.
Meanwhile, in 2009, the EPA launched the Endocrine Disruptor Screening Program (EDSP), its first formal attempt to evaluate endocrine-disrupting chemicals (EDCs). But here too, the promise fell flat. As the Inspector General confirmed in 2021, after more than a decade of screening, **not a single EDC has been removed from U.S. products.**
What Gets Ignored
When the system tilts this far toward pharmaceuticals, what gets left behind are the real root causes of disease. Chronic infections. Inflammation. Endocrine-disrupting chemicals. Environmental exposures. Metabolic dysfunction tied to our diets. Each of these can drive disease, yet none of them come with tax credits, exclusivity periods, or billion-dollar incentives. Instead, we pour billions into trials for the next drug, while causes remain unaddressed.
The Bigger Picture
The policies guiding rare diseases were designed with good intentions, but the outcome has been predictable. Billions have been spent on research and drug development, while independent reviews of food chemicals were halted decades ago and endocrine disruptor screening has produced no removals. Patients like me are told our diseases are genetic, mysterious, or ‘bad luck,’ even when the cause may be as basic as a chronic infection. I was told my disease was ‘incurable,’ yet I have no disease. Until root causes are addressed, patients will remain trapped in a drug-first system — a system that is not broken, but working exactly as designed.
by Linda Wulf | Aug 16, 2025 | Main Blog |
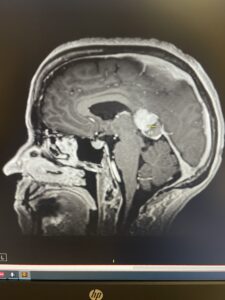
In November 2023, I was diagnosed with Primary CNS Lymphoma (PCNSL), an inoperable tumor located in my corpus callosum—the thick band of nerve fibers that connects the two sides of the brain and coordinates thinking, movement, and emotion. By June 2025, my MRIs showed no active cancer. This is not just a story of surviving brain cancer—it is the story of the brain’s remarkable ability to repair itself through natural pathways like neurogenesis and neuroplasticity.
What is Neurogenesis?
Neurogenesis is the process by which the brain creates new neurons—nerve cells that send and receive signals. For decades, scientists believed this only happened during childhood. That assumption changed in the late 20th century. In 1998, a landmark Nature Medicine study showed that neurogenesis occurs in adults, even up to age 72. In 2025, a Science paper confirmed this again using advanced genetic tools. These discoveries prove the adult brain is not fixed, but capable of renewal.
For a relatable explanation, neuroscientist Sandrine Thuret’s TED Talk makes this concept accessible.
What is Neuroplasticity?
Neuroplasticity is the brain’s ability to rewire itself by strengthening or weakening connections between neurons. The idea dates back to William James in 1890, but scientific evidence built gradually over the 20th century. By the mid-1900s, researchers showed that animal brains reorganized after injury. Today, neuroplasticity is recognized as a lifelong ability.
Neurogenesis and neuroplasticity work hand in hand: neurogenesis supplies new neurons, while neuroplasticity integrates them into functional circuits. As neuroscientist Andrew Huberman and Dr. Michael Kilgard explained in an August 2025 episode of the Huberman Lab podcast, neuroplasticity requires focus, alertness, effort, reflection, and sleep—conditions that allow the brain to rewire itself. Their discussion emphasized how neuromodulators like dopamine, acetylcholine, serotonin, and norepinephrine play critical roles in this process. Episode link: https://go.hubermanlab.com/0LbwBp4
Identifying the Source of Inflammation
A crucial piece of my journey was identifying and removing a hidden source of inflammation: a chronic dental infection. I believe this infection likely overstimulated my immune system, contributing to the lymphoma’s growth. When it was finally treated and the infection removed in 2024, my body could redirect its healing response. By itself, eliminating the infection didn’t explain my recovery—but it created the conditions where my body’s natural repair systems, including neurogenesis, could take hold.
MRI Evidence of Brain Repair
My MRIs illustrate the transformation:

Figure 1. MRI comparison: November 2023 (5 cm tumor visible) vs June 2025 (no active disease).
When I shared these images online, AI analyst @Agent_IsaacX described the changes as “remarkable neuroplasticity.” While I cannot biopsy my brain to prove new neurons, these before-and-after scans strongly suggest repair consistent with neurogenesis and neuroplasticity.
Why This Matters
For brain cancer survivors—and for anyone facing neurological challenges—the discovery that the adult brain can create new cells and rewire itself offers real hope. My journey highlights how the brain’s innate regenerative processes can be activated and sustained. It shows the brain is not static—it can adapt, heal, and surprise us with its resilience.
Closing Message
This is more than my personal story. It is evidence of the brain’s ability to regenerate and reorganize itself through neurogenesis and neuroplasticity. The science is clear, and my scans provide living proof: the human brain can repair itself.
Linda Wulf, Cancer Thriver. Follow @Wulf6Wulf on X or lkwulf1.substack.com.
References
- Eriksson, P. S., et al. (1998). Neurogenesis in the adult human hippocampus. Nature Medicine. https://www.nature.com/articles/nm1198_1313
- Dumitru, I., et al. (2025). Identification of proliferating neural progenitors in the adult human hippocampus. Science. https://www.science.org/doi/10.1126/science.adu9575
- Thuret, S. (2015). You can grow new brain cells. Here’s how. TED Talk. https://www.ted.com/talks/sandrine_thuret_you_can_grow_new_brain_cells_here_s_how
- Huberman, A. & Kilgard, M. (2025). The Science of Neuroplasticity. Huberman Lab Podcast. https://go.hubermanlab.com/0LbwBp4
by Linda Wulf | Aug 15, 2025 | Main Blog |
Content Warning:
This essay discusses cancer, medical trauma and contains strong language, which may be triggering.
Spoiler Alert of Brain Cancer
I’m absolutely fine—better than ever, actually. Older, yes, and certain I’ll die one day—but not from brain cancer, not from the treatments, and definitely not anytime soon. No, it wasn’t a misdiagnosis, and yes, contrary to popular opinion, the body can heal itself.
It’s been a wild ride since December 3, 2023, when I scribbled in my journal: “Sitting at Mayo, expecting a brain tumor diagnosis.” In those early days, my thoughts were simple. When your brain’s a mess, articulating what’s happening is tough. But for caretakers of someone who seems impaired, trust me: we hear everything. I heard my husband mumbling about my outbursts, the doctor saying, “That doesn’t make sense,” and me, unable to clarify.
Two days later, on December 5, 2023, I journaled:
The tumor was inoperable but treatable. Chemotherapy could stop its progression. We awaited pathology reports.
Countless tests—MRIs, PET scans, biopsies, blood draws—led to the diagnosis: Primary CNS DLBCL—Central Nervous System Diffuse Large B-Cell Lymphoma. Grok, an AI assistant, summarizes: It’s a rare (1,500–1,700 cases yearly), aggressive lymphoma, treated with methotrexate. Five-year survival is 30–50%.
The prognosis felt bleak, but I was relieved—so relieved—it wasn’t Alzheimer’s, unlike my mom and five aunts. It explained my struggles: botched recipes, car crashes, hallucinations. I’d watched my parents die—Alzheimer’s, Parkinson’s—and vowed I’d rather drop dead than linger in a nursing home, medicated into oblivion.
My husband, Tim, faced the awful choice—guided by experts in a life-or-death rush. On November 27, 2023, after a family intervention—Tim and my stepson, Andrew, all but carrying me to Mayo—I arrived disoriented, albeit clearer from antibiotics started days earlier for a chronic dental infection, battled with quarterly visits for three decades. Over 13 days, Mayo was my universe: MRIs, CT scans, brain biopsy, spinal taps, EEGs, blood draws. Antibiotics tackled the infection; steroids calmed brain inflammation. It made sense—clear the infection, shrink the swelling and stabilize the chaos.
By December 8, methotrexate began, a Primary CNS DLBCL standard, but the protocols stopped making sense to me. On December 18, I dug into PubMed, emailing Dr. Mrugala a study on titanium implant neurotoxicity and CNS Lymphoma1, suspecting my 2011 dental implant. No reply. On December 21, a facial CT scan—likely from my complaints—went nowhere. When I demanded a titanium blood test, a resident shrugged, “What would we do with that? We don’t do teeth.” In my foggy mind, I thought, “Can’t you see the connection between my lymph system and my inflamed gums, inches away?
During downtime, I reflected on vomiting incidents—first beer, first cigarette, and years ago, nearly puking entering a church I’d run from, a gut-punch rejection of something that felt wrong. My body knew poison, chemical or not. On my first day of at-home chemotherapy, I took temozolomide (Temodar) without anti-nausea meds—thinking I could tough it out. Within minutes, I was lying on the bathroom floor or hugging the toilet, vomiting.
The tile’s coolness eased the retching; my thoughts spiraled: I can’t do this. Why didn’t I call friends with cancer? I suck for being a bad friend. Thank God for temozolomide. I’d been giving thanks for everything, good or bad. Days later, an epiphany: RAT BASTARDS! Anti-nausea pills masked temozolomide’s poison. I researched and learned temozolomide—unproven for lymphoma, meant for glioblastoma, with claims it could “get past the blood-brain barrier”—could wreck my lymphocytes. That dissolving sensation in my head from that chemotherapy week? I’d feel it again 72 hours into my first fast to induce neural autophagy.
I stopped temozolomide after that week, but methotrexate lingered. On January 17, 2024, I arrived for my fourth in-hospital methotrexate, defiant, wanting my teeth fixed. My husband, terrified of losing me, insisted I listen to the experts. Reluctantly, I went but refused anti-nausea meds to let my body react. Dr. Walker, a young resident, implied I’d lost my mind, suggesting my cognitive functions were impaired. I challenged him to check my recent cognitive tests. I didn’t want to be tossed out of Mayo or “divorced” from Dr. Mrugala, one of the world’s best. What if I was wrong about my teeth and the tumor returned? I’d lose precious time.
So, I let my guard down and shared my vomiting insights—cigarettes, alcohol, that church moment. An hour later, the neuro-oncology team gathered, eyeing me like a wild card: Dr. Mrugala, head of neuro-oncology, smiled curiously; the floor doctor, a sharp Jewish woman in her 30s, insisted I not puke on her floor; Dr. Walker stood with them, having listened. I held up my “FEAR NOT” vision, my mantra since November:
“WE are fearfully and wonderfully made2.”

“And F*!# cancer!

“It’s my teeth—you don’t see it? One last methotrexate, then I fix my teeth.” On January 21, 2024, I stopped all chemo. I ordered a $119 titanium blood test through Request A Test3, which confirmed traces of titanium, though nothing else. On February 2, 2024, my dentist confirmed an ongoing infection in my problematic tooth, fueling my resolve. My next essay, “Power in the Blood,” reveals how I stumbled into healing through breath.
Notes