by Linda Wulf | Jun 10, 2025 | Chemical Exposure Unleashed, Main Blog |
Introduction: A Personal Awakening
I once believed that products approved by the U.S. Food and Drug Administration (FDA) were safe for use. But after my own battle with cancer, that illusion shattered. With the help of Grok AI and Chatbot GTP, I discovered that 7,157 unique chemicals—many of them untested or inadequately reviewed—persist in our food, drugs, packaging, and personal care products. This article is not just a critique of the FDA’s structure and oversight—it is a call for reform grounded in lived experience, regulatory history, and emerging science.
The FDA’s Flawed Governance: Overlapping Databases
The FDA’s governance is riddled with fragmentation, weak authority, and a troubling reliance on industry self-regulation. Responsibilities are split among the Center for Food Safety and Applied Nutrition (CFSAN), the Center for Drug Evaluation and Research (CDER), and a lightly managed oversight of cosmetics. The agency functions more like a passive archive for industry-submitted documents than an active safety watchdog.
The cornerstone of this problem is the ‘Generally Recognized as Safe’ (GRAS) process[1]. Originally intended to streamline approval of common food ingredients, GRAS allows substances to be deemed safe based on scientific evidence or expert consensus, often without FDA review. Today, companies can self-affirm safety by hiring their own experts, bypassing rigorous FDA oversight. Of the 3,970 substances in the FDA’s Substances Added to Food Database[2], roughly 3,670 have never undergone active FDA safety assessments.
Industry influence compounds the problem. From 1998 to 2025, the ultraprocessed food industry spent $1.4 billion lobbying, while pharmaceutical firms spent $6.3 billion. These efforts have blocked reform—most notably the closure of the GRAS self-affirmation loophole[3], which remains open despite HHS Secretary Robert F. Kennedy Jr.’s March 2025 directive to eliminate it[1].
Historical Context and Regulatory Evolution
When the FDA was created in 1906 under the Pure Food and Drugs Act, the food supply contained few synthetic chemicals. The agency’s early mission focused on preventing adulteration and mislabeling. With the 1938 Federal Food, Drug, and Cosmetic Act, the FDA was empowered to conduct drug safety reviews. The 1958 Food Additives Amendment created the GRAS exemption[3], initially listing about 700 substances.
Over the decades, the GRAS process grew to include thousands of additional substances through self-affirmation. In 1997, a voluntary notification system further weakened FDA’s oversight. Parallel gaps in cosmetics, OTC drugs, and supplements evolved similarly. The result: 7,157 unique chemicals now permeate the U.S. consumer landscape, many without adequate federal oversight.
The Scale of Chemical Exposure
The FDA maintains several overlapping databases, each detailing different chemical categories:
- Substances Added to Food (3,970 substances): Includes sodium benzoate, which can form carcinogenic benzene when combined with ascorbic acid[4], and monocalcium phosphate, a leavening agent linked to health risks from high phosphorus intake[5].
- Inventory of Food Contact Substances (3,652 substances)[7].
- Select Committee on GRAS Substances (SCOGS): 451 entries (370 substances), including carrageenan, linked to inflammation and cancer risks[8].
- Inactive Ingredients Database (9,196 entries, 1,048 unique substances): Includes propylparaben, an endocrine disruptor[9].
- Dietary Supplement Label Database (205,782 labels): Includes ascorbic acid, which poses risks of overexposure in self-affirmed uses[10].
These databases often rely on industry data, not independent review, leaving Americans exposed to substances never meaningfully tested for long-term safety.
The Hidden Health Risks
Of the 7,157 unique chemicals in circulation, many are endocrine disruptors—compounds that interfere with hormonal systems[11]. These are linked to obesity, infertility, diabetes, and even cancer. Research suggests up to 20% of food additives may have endocrine-disrupting properties[12].
Notable examples:
- Propylparaben in drugs and lotions mimics estrogen and may impair reproductive health[13].
- Carrageenan used in food and toothpaste contributes to colitis and chronic inflammation[14].
- Sodium benzoate, still legal, may generate carcinogenic byproducts when paired with vitamin C[6].
Consumers often assume FDA approval means safety, but unless they dig into obscure databases or advocacy sites, they’re unaware of these systemic risks.
Overlapping Oversight and Personal Care Risks
Personal care products pose unique risks. Ingredients like propylparaben, commonly found in creams and lotions, are absorbed through the skin. Despite their systemic impact, cosmetics remain under-regulated. No dedicated FDA database exists to track their safety, perpetuating exposure through regulatory neglect.
Seeking Transparency
For proactive consumers, tools exist but require effort. The Inactive Ingredients Database (IID)[9] and apps like Yuka[15] allow users to check ingredient profiles, but they lack plain-language risk summaries. Meaningful transparency will require integrating FDA databases into a single, user-friendly platform.
A Call for Comprehensive Reform
To protect public health, the FDA must:
- Consolidate CFSAN, CDER, and cosmetic oversight into one cohesive regulatory body.
- Eliminate GRAS self-affirmation and require FDA review of all safety claims.
- Limit industry lobbying and funding of research tied to regulatory decisions.
- Create a single, public database of all approved chemicals with searchable summaries.
- Despite evidence of harm, unsafe substances often remain in use for years due to slow regulatory action, perpetuating public health risks.
- Immediately restrict high-risk substances like Red No. 40[16], and propylparaben instead of phasing them out over the years.
Until reform arrives, Americans must become their own watchdogs—researching the safety of food, medication, personal care products, and even their tap water.

by Linda Wulf | Mar 24, 2025 | Main Blog |
Imagine picking up a snack from the grocery shelf—crackers, soda, or a candy bar. You scan the ingredients, spotting familiar names like sugar or salt, and maybe some tongue-twisters like “sodium benzoate” or “xanthan gum.” What you don’t see is a quiet stamp of approval baked into U.S. law: “Generally Recognized as Safe,” or GRAS. It’s a term most of us have never heard of, yet it governs thousands of substances in our food. Enshrined in regulation since 1958, GRAS was meant to keep us safe. But over decades, it’s morphed into a system so entrenched that we’re consuming additives daily—some of which might be silently harming us, and we don’t even know it.
What Is GRAS?
GRAS stands for “Generally Recognized as Safe,” a designation created by the U.S. Food and Drug Administration (FDA) to classify food ingredients that don’t need rigorous premarket testing. If a substance is widely accepted by qualified experts as safe—based on scientific data or a long history of use—it gets the GRAS label and can go straight into your food. Think of it as a fast-pass lane: sugar, vinegar, and spices sailed through because they’ve been used forever, while new chemicals can qualify with enough expert agreement.
The idea sounds reasonable—why bog down the FDA with red tape for stuff everyone trusts? But here’s the catch: GRAS isn’t just a handful of pantry staples. It’s thousands of additives—flavorings, preservatives, thickeners—slipped into processed foods, from your morning cereal to your evening takeout. And as the system evolved, the question shifted from “Is this safe?” to “Who’s even checking?”

How It Started: The 1958 Food Additives Amendment
Generally Recognized as Safe (GRAS) was born in a simpler time. Before the 1950s, the FDA had broad authority to police food safety, but the rise of synthetic chemicals—think artificial flavors and stabilizers—overwhelmed the system. In 1958, Congress passed the Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act, demanding premarket approval for new additives unless they were GRAS. The law defined GRAS as substances “generally recognized, among experts qualified by scientific training and experience,” as safe based on science or common use before 1958.
Back then, the FDA took the lead. Companies petitioned the agency to affirm ingredients like caffeine or citric acid as GRAS, and the FDA published its rulings in the Code of Federal Regulations. It was a manageable list, with the agency carefully vetting each substance. The goal? Protect consumers while letting innovation flow. For a while, it worked.
How It Evolved: From Oversight to Loophole
Fast-forward to the 1990s, and the cracks started showing. The FDA, swamped with petitions and short on resources, proposed a game-changer in 1997: ditch the formal affirmation process for a voluntary “notification” system. Companies could now self-affirm a substance as GRAS, send the FDA their safety data (or not), and start using it unless the agency objected. By 2016, this became official policy. The shift was seismic—oversight moved from the FDA’s desk to industry boardrooms.
The numbers tell the story. In the petition era, it’s estimated that fewer than 400 substances earned FDA-affirmed GRAS status, though exact figures are hard to confirm without historical records. Today, a database of GRAS substances downloaded in July 2024 lists exactly 3,972 entries—covering everything from vanilla extract to obscure flavorings like “(+/-)-2-Methyltetrahydrofuran-3-thiol Acetate.” These additives touch nearly every processed bite you take.
Enshrined in Law, Embedded in Our Lives
That 1958 amendment enshrined GRAS as a legal fixture, and its evolution has made it untouchable. Companies love it—no lengthy approvals, no mandatory FDA sign-off. The FDA leans on it too, stretched thin by budget cuts and a flood of new ingredients. But what about us, the eaters? We’re left trusting a system where safety is assumed until proven otherwise—sometimes decades too late.
Take partially hydrogenated oils, commonly known as trans fats: GRAS for years, they clogged arteries until science linked them to heart disease in the 1990s. The FDA didn’t ban them until 2015, with a full phase-out by 2021—20 years of delay while we ate. Or consider caffeine-spiked alcoholic drinks like Four Loko, yanked in 2010 after ER visits spiked, yet similar combos still linger in loopholes. These aren’t outliers; they’re symptoms of a system that waits for harm to scream before it whispers “stop.”
The Unseen Cost
Here’s the kicker: we don’t know what’s killing us because GRAS hides in plain sight. Some additives—like ethylene oxide, a known carcinogen, or alkanet root extract linked to liver toxicity—were once deemed GRAS by the Flavor and Extract Manufacturers Association (FEMA) but have since been stripped of that status due to safety concerns. Yet they may still linger in our food if companies self-affirm their safety because the FDA’s hands are tied. Worse, no one tracks how many of these 3,972 additives a body can absorb or what happens when they combine—like drugs, they have effects that linger, burdening the body’s cleanup systems, yet they’re tested in isolation, not as the chemical cocktail we consume.
This self-affirmation loophole even extends to the vitamin and supplement market, where untested compounds can slip into products we assume are safe. And that’s just food—add in exposures from over-the-counter drugs, pharmaceuticals, and beauty products (which the FDA barely regulates, leaving safety to companies), and the disconnect grows: your body doesn’t care about regulatory silos; it processes the total exposure.
Meanwhile, new science—like the Human Microbiome Project’s discoveries since 2007 showing how additives can disrupt gut bacteria linked to health—reveals risks the sluggish GRAS system can’t keep up with: how can regulation protect us when it lags decades behind what we’re learning about our bodies? Industry self-policing means no one’s compelled to pull the plug until the evidence is overwhelming, and even then, it’s a slog. We’re consuming a chemical cocktail daily, legally enshrined, and most of us don’t even know what GRAS stands for.
Conclusion
This is just the beginning. GRAS started as a safeguard, evolved into a loophole, and now sits as a silent giant in our food system. Next time you grab a snack, ask yourself: who decided this was safe—and how long will it take to find out if they were wrong? More importantly, what is your body telling you?
Explore our curated resources for in-depth insights, expert guidance, and valuable tools to expand your knowledge and stay informed.
by Linda Wulf, with assistance from Grok (xAI)

by Linda Wulf | Mar 5, 2025 | Main Blog |
Our daily lives are steeped in chemicals—from the preservatives in your cereal to the synthetic colors in your lipstick. Harmful additives shape what we eat, slather on our skin, and absorb into our bodies, often combining in a “cocktail effect” that amplifies their risks. In the U.S., many of these substances are greenlit as safe, yet the EU bans them outright, citing links to cancer, hormonal chaos, or developmental harm. Whether it’s your sandwich or your sunscreen, tools like the YUKA app and AI platforms like Grok are here to help us decode the danger and reclaim control.
Unraveling the Risks of Harmful Additives
Your grocery cart tells only half the story. Sodium benzoate spikes your soda, Yellow 5 dyes your candy, and titanium dioxide whitens your gum—additives the FDA calls “generally recognized as safe” (GRAS). Meanwhile, the EU disagrees, banning or restricting them as harmful additives tied to serious health risks. Potassium bromate in bread? Cancer concerns. BHA in snacks? Endocrine disruption. The U.S. shrugs; Europe acts. But it’s not just food. Your bathroom shelf is loaded, too—parabens in lotions, phthalates in nail polish, formaldehyde releasers in mascara. These sneak through your skin, a direct highway to your bloodstream; no digestion is required.

This chemical flood doesn’t sit still. Harmful additives from food and cosmetics mingle daily—think BHT from your moisturizer meeting Yellow 5 from your lunch. Bioaccumulation kicks in; some linger, stacking up over decades. Your body’s quirks—metabolism, hormones, age—twist the impact further. The FDA tests additives solo, but who lives solo? Add in medications, over-the-counter remedies, or that scented body cream, and the mixture toxicity looms large—a gap science hasn’t bridged. From plate to face, harmful additives are everywhere, and the stakes are personal.
Why We Need a New Approach
We’re guinea pigs in a slow-burn experiment. Research lags, stuck on single-ingredient studies while we marinate in a chemical stew. The EU’s “ban first” ethos—axing parabens or titanium dioxide for potential DNA damage—clashes with the U.S.’s “use until proven guilty” GRAS stance. Formaldehyde in your hair straightener? Still legal here but banned there. This divide screams for real-world testing: food plus cosmetics, long-term, all at once. Until then, policy creeps, leaving us exposed.
Awareness is our edge. If we grasp why Europe rejects what America accepts—harmful additives in our burgers and our blush—we can push back. It’s not just about laws; it’s about knowing enough to dodge the risks today.
Taking Control: Dodging Harmful Additives
You’ve got power here. In the kitchen, whole foods—an orange over a dyed snack—slash harmful additives cold. Cooking at home? You call the shots; no mystery stabilizers are allowed. Labels are gold—spot BHA in food or phthalates in lotion, both flagged abroad for a reason. Organic cuts some synthetics (in produce and skincare), though it’s not a cure-all. Local markets mean fresher food and often cleaner creams—less preservation, fewer chemicals.
Beyond food, rethink your routine. Swap bottled water (plastic leachables) for a filter. Check meds—artificial fillers pile on. Your makeup bag? Ditch parabens and triclosan—harmful additives banned in the EU but lurking in U.S. tubes. Grow herbs or mix a DIY balm to skip store-bought unknowns. Rotate what you eat and apply—this dilutes the load.
Tech seals it. YUKA scans your groceries and some cosmetics, flagging harmful additives like Yellow 5 or formaldehyde in a heartbeat. Grok digs deeper—why’s the EU nixing what the U.S. shrugs at, from bread to body lotion? These tools turn confusion into clarity, making every choice a stand for your health.
A Healthier Future, One Choice at a Time
Harmful additives aren’t just in your fridge—they’re in your foundation, your face cream, and your life. The U.S.-EU split proves “safe” is a guess, not a guarantee, and that cocktail effect—food meeting cosmetics—ups the ante. Step one: Know it. Step two: Act—lean on YUKA for quick hits, Grok for the why, and your gut to filter the rest. This isn’t fear; it’s balance, tilting toward health over convenience.
Looking Ahead
More’s coming. We’ll explore how YUKA, Grok, and other digital allies are rewriting transparency—real-time intel on harmful additives from your plate to your powder. Stay tuned.
by Linda Wulf | Jan 14, 2025 | Main Blog |
Oxygenation
- Increased Oxygen Supply: Deep breathing has allowed me to feed my cells with more oxygen, potentially enhancing the efficiency with which my mitochondria convert this oxygen into ATP, the energy currency of our cells. This could explain the noticeable surge in my energy levels and physical capabilities.
Stress Relief
- Lowering Oxidative Stress: Through deep breathing, I’ve managed to lower my stress levels, reducing cortisol which in turn might decrease oxidative stress on mitochondria. Cortisol is a stress hormone, and high levels can lead to cellular damage. By managing stress, deep breathing might promote mitochondrial repair and prevent further damage.
Circulation
Autophagy Enhancement
-
Supporting Cellular Cleanup: While fasting is known to kick-start
autophagy, a process where cells break down and recycle their components, I speculate that deep breathing could enhance this process, particularly mitophagy, where damaged mitochondria are cleared out. This might be playing a role in my health improvements.
pH Balance
-
Optimal Cellular Environment: By controlling CO2 levels through my breath, I might be maintaining the ideal pH for mitochondrial enzymes to work efficiently, ensuring they function at their best. pH balance is crucial for enzyme activity within cells.
Avoiding Modern Toxins
Holistic Health Synergy
Conclusion
While the scientific community has yet to fully investigate these connections in humans, my personal experience supports the hypothesis that a holistic approach—combining deep breathing, fasting, and clean living—can enhance mitochondrial health. Deep breathing goes beyond mere relaxation; it’s a fundamental method to nurture cellular health. Every breath has the potential to recharge cells at their core. Although further research is essential to validate these observations, the practice appears to have significantly contributed to my healing journey, enriching both my life and my cells in ways that suggest deep breathing might indeed revitalize mitochondria.
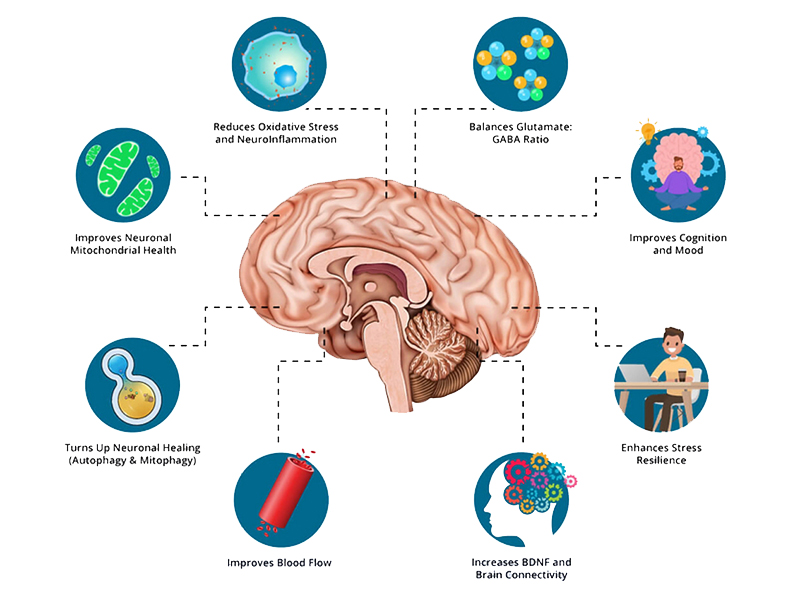
by Linda Wulf | Dec 15, 2024 | Main Blog |
What is Autophagy?
Autophagy, derived from the Greek words “auto” (self) and “phagy” (eating), is a physiological process where cells dismantle and recycle their own damaged or unnecessary components. This self-cleaning mechanism is essential for:
- Removing damaged organelles or proteins: By clearing out potentially harmful cellular debris.
- Recycling nutrients: Providing cells with essential building blocks during nutrient scarcity, such as during fasting.
Neural Autophagy
- Neural autophagy specifically refers to this process within neurons, focusing on the brain’s unique needs for cellular cleanup:
- Neurons rely on autophagy to manage protein aggregates, damaged mitochondria, and other cellular waste that can accumulate due to high brain activity.
Neural Autophagy is thought to protects against neurodegenerative diseases: By clearing out toxic protein aggregates, neural autophagy might help prevent or mitigate conditions like Alzheimer’s, Parkinson’s, and Huntington’s disease.
How Fasting Induces Autophagy
- Fasting triggers autophagy as the body responds to nutrient deprivation.
- Energy Conservation: The body conserves energy by recycling cellular components.
- Cellular Repair: Autophagy increases to repair and maintain cellular integrity, especially beneficial for neurons which have a limited regeneration capacity.
Considerations for Fasting for Neural Autophagy
When considering fasting for the benefits of neural autophagy, here are some key points:
- Start with Shorter Fasts: Begin with 16-24 hours to assess your body’s response. This can be part of intermittent fasting protocols where autophagy begins to increase significantly around 12 to 16 hours into fasting, with neuronal benefits possibly starting around 24 hours.
- Progress to Longer Fasts: For more pronounced effects, some advocate fasting for 3 days (72 hours), where autophagy might be significantly enhanced, potentially aiding in cellular repair and cognitive function. However, extended fasting should be approached with caution.
- Extended Fasting: There’s mention of fasts from 36 hours to 3 days for maximal activation of chaperone-mediated autophagy, a specific type beneficial for neural health.**
- Hydration: Staying hydrated is crucial, particularly during longer fasts, to support bodily functions.
- Consultation: Before engaging in longer fasting periods, especially with pre-existing health conditions, consulting with a healthcare provider is wise to tailor fasting to your health needs.
Remember, the optimal fasting duration for neural autophagy can vary greatly among individuals based on health goals, current health status, and lifestyle. Personal experimentation under safe conditions, or better yet, under medical supervision, is advised to determine what works best for you.
Duration and Continuation of Autophagy
Once initiated, the duration of elevated autophagy depends on:
- Fasting Duration: Autophagy can start within hours and might continue as long as the fasting lasts or until nutrients are replenished. Peak activity might occur around 24 to 72 hours.
- Nutrient Intake: Eating, particularly a meal high in nutrients or protein, signals the body to downregulate autophagy, shifting from breakdown to building processes.
- Cellular Needs: Autophagy continues as long as there’s a need for cellular cleanup or repair.
- Physiological or Pathological Conditions: Chronic conditions or aging might alter baseline autophagy levels, influencing how long and how effectively autophagy functions.
- Genetic and Environmental Factors: These can affect the efficiency and duration of autophagy.
In summary, while fasting can promote neural autophagy, the exact timing and duration of this process are highly individual and depend on a variety of factors including the fasting protocol, the individual’s metabolic state, and the reintroduction of nutrients. Always approach fasting with a balanced perspective on health, ensuring it complements other health practices rather than standing alone.
By: Linda Wulf and Grok 2
Click here for more information on Autophagy and Fasting. If you want to learn more about autophagy and fasting, feel free to reach out to us! We are here to provide you with the latest research, tips, and guidance on how fasting can activate autophagy and improve your overall health.

by Linda Wulf | Dec 13, 2024 | Main Blog |
Gadolinium, a rare earth metal, serves as a contrast agent in MRI scans, enhancing the visibility of internal structures. This enhancement is crucial for diagnosing various conditions, including cancers, by providing clearer images of organs, tissues, and blood vessels.
The process of an MRI with gadolinium contrast, as applied in my case for diagnosing CNS Lymphoma, involves multiple non-contrast scans initially. These scans provide a baseline view of the brain’s structure. Following this, gadolinium is administered through an IV.
Gadolinium’s purpose is to enhance areas where the blood-brain barrier might be disrupted, such as in tumors, by making them appear brighter on MRI images. This contrast injection happens after initial scans are complete, allowing for comparison between the pre-and post-contrast images, which is crucial for identifying active disease areas. The repetition of this process over a week in my case was presumably to monitor rapid changes or responses to initial treatments, though the necessity of such frequency remains a point of personal contention and medical curiosity.
My experience with gadolinium was not through standard diagnostic procedures but was thrust upon me due to an urgent diagnosis of Central Nervous System Lymphoma (CNS Lymphoma), a rare form of brain cancer. This critical situation, treated at a premier medical research center, necessitated three MRIs with gadolinium contrast within my first week.
The FDA’s directive suggests that in such scenarios, the medical urgency might supersede the standard protocols for obtaining informed consent for gadolinium administration, highlighting how exceptional circumstances can dictate medical decision-making. At the time, I was unaware of the possible adverse side effects of gadolinium.
Gadolinium Poisoning
The pivotal moment in my understanding came several months ago during a casual conversation with a medical technician as I was being prepared for another MRI. He casually mentioned that gadolinium, the contrast agent used in my scans, is a rare earth element.
This sparked a deep dive into research, unveiling the potential long-term effects of gadolinium retention. Gadolinium poisoning, or gadolinium deposition disease, presents risks often under-discussed, with symptoms ranging from skin thickening to neurological issues like brain fog, muscle pain, and cognitive impairments. These symptoms might not manifest immediately but can emerge over time, particularly with repeated exposure, underscoring the necessity for cautious use and monitoring. Gadolinium deposits have been found in the brain.
Having just removed lymphoma from the center of my brain, the idea of introducing more elements into that now vulnerable area seemed counterintuitive to me. Despite reassurances from my doctor, the absence of routine testing for gadolinium levels in patients like myself, especially in a facility conducting trials on gadolinium exposure with non-patient participants, felt like a betrayal of trust.”
Betrayal of Trust
This was compounded by the FDA’s 2018 Drug Safety Communication regarding gadolinium, which was intended to inform healthcare professionals and patients about the risks associated with gadolinium-based contrast agents (GBCAs). A review of the of document below reveals this communication was poorly structured, inconsistent, and unclear. It includes the following paragraph1:
“All MRI centers should provide a Medication Guide the first time an outpatient receives a GBCA injection or when the information is substantially changed. In general, hospital inpatients are not required to receive a Medication Guide unless the patient or caregiver requests it. A health care professional who determines that it is not in a patient’s best interest to receive a Medication Guide because of significant concerns about its effects may direct that it not be provided to that patient; however, the Medication Guide should be provided to any patient who requests the information. (emphasis in bold is mine).
This lack of clarity not only undermines informed consent but also erodes the trust patients place in medical institutions and especially in regulatory bodies.
Things I could do…armed with knowledge, I took charge of my health. The decision to forgo further chemotherapy, focusing instead on resolving dental issues as a suspected root cause for my cancer was met with resistance. My insistence on understanding my gadolinium levels through a urine test marked my final and transition from patient to advocate. The results were alarming, prompting a reevaluation of my treatment plan and a decision to not undergo further exposure to gadolinium.
My experience underscores a critical need for transparency in medical practices and the competency of government agencies. Patients deserve not just treatment but also understanding—not just diagnosis but dialogue. The medical practice must evolve to include routine monitoring for gadolinium retention, especially in patients undergoing multiple scans. Moreover, the discussions around medical procedures should include not only benefits but also potential risks.
Conclusion
In conclusion, this essay isn’t just a recount of personal medical trials but a call to action for healthcare reform. As we advance in medical technology, let us not forget the human element, ensuring that the tools meant to heal do not inadvertently harm. My story with gadolinium is one of many, advocating for a future where medical care is as transparent as the images it seeks to produce.
- FDA Drug Safety Communication: FDA warns gadolinium-based contrast agents (GBCAs) are retained in the body. May 16, 2018. Accessed: -09/24/2024 https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body
Additional References Consulted:
Qu H, Li W, Wu Z, et al. Differences in hypersensitivity reactions and gadolinium deposition disease/symptoms associated with gadolinium exposure to gadolinium-based contrast agents: new insights based on global databases VigiBase, FAERS, and IQVIA-MIDAS. BMC Med. 2024;22:329. https://doi.org/10.1186/s12916-024-03537-2
Yao X, Hu J, et al. Deposition of gadolinium in the central and peripheral nervous systems and its effects on sensory, cognitive, and athletic implications after multiple injections of gadolinium-based contrast agents in rats. Am J Neuroradiol. 2024;45(8):1153-1161. DOI: 10.3174/ajnr.A8295.
Multihance (gadobenate dimeglumine). Princeton, NJ: Bracco Diagnostics Inc; 2024. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/021357s027,021358s025lbl.pdf
NOTE: When I sought urine tests to measure gadolinium levels, my doctors were unwilling to order them, relying instead on a mathematical calculation they believed sufficient. Determined to understand my body’s gadolinium burden, I purchased the tests myself online — item 3527, Comprehensive Urine Element Profile. This test also offers valuable insight into 33 elements, including lead, mercury, aluminum, arsenic, iron, zinc, calcium, and more. I’ve had success with True Health Labs (https://truehealthlabs.com) for ordering and processing these tests at home. Read the instructions carefully — it takes some planning. These tests are not covered by insurance or Medicare.
An “acceptable” gadolinium level is generally considered to be ≤ 0.019 mcg/g creatinine, though ranges may vary slightly by lab. My last MRI with contrast was on July 16, 2024. Since then, I’ve undergone three separate urine analyses to track my gadolinium levels over time:
Gadolinium Test Results
– August 14, 2024: 11.664 mcg/g creatinine – Severely elevated
– November 5, 2024: 0.500 mcg/g creatinine – Improved, but still high
– June 16, 2025: 0.25 mcg/g creatinine (24-hour collection) – Within normal range per lab standards
This trajectory has been encouraging. The downward trend suggests that my body is slowly clearing the gadolinium burden, possibly aided by lifestyle changes, detox protocols, or simply time. However, contrary to common assumptions, these foreign substances can remain in the body far longer than expected, and the full extent of their potential impact on human health remains uncertain. This journey of self-advocacy has been eye-opening, and I share these details to empower others navigating similar challenges.