My Cancer Protocol – And Walked Away
Midnight, January 2024, Mayo Clinic room. I was sitting in my room at the Mayo Clinic, still recovering from another high-dose methotrexate infusion. The side effects were accumulating—brain fog, weakness, immune depletion—but the unease that grew inside me wasn’t just physical. It was something deeper, something instinctual, something that wasn’t right. That night, I opened my laptop and typed a question into ChatGPT: “Who writes the guidelines for central nervous system lymphoma?” The answer came back quickly: the National Comprehensive Cancer Network (NCCN)—a non-profit alliance of elite cancer centers across the United States that produces the protocols used in nearly every major institution. The same guidelines that were directing my care.
When I visited the NCCN website that night, something unexpected appeared. At the top of the guidelines submission page, I saw the names AbbVie, Genentech, and Pfizer. The wording implied that these companies weren’t just supporters but gatekeepers—positioned to influence which drugs and protocols made it into the standard of care, long after the research was done and the manufacturing complete. Moments later, the page shifted—replaced by a list of credentialed researchers from major institutions like Stanford, Harvard, and Mayo Clinic. But that fleeting glimpse revealed a deeper truth: the protocols steering my care appeared, if only briefly, to answer to industry interests—not to independent science.
That was the decisive moment it changed for me.
When the Disconnect Became Clear
My decision to walk away didn’t begin with ChatGPT, or NCCN, or even the protocols. It started weeks earlier, on December 5, 2023, when I first heard the phrase “inoperable but treatable.” I remember writing in my journal that day and feeling something crack: a disconnect between what I knew about nature, healing, and inflammation—and what the doctors were saying. They told me this cancer had no cause and no cure, but showed some enthusiasm about how we hope to figure it out soon, almost like a pep talk to join the “let’s beat cancer team.”
It took a little over a month for me to exit the program, but it turns out, I am not a team player. I knew my body’s story: I’d lived with a chronic dental infection, which was active at this time. What they offered wasn’t hope; it was protocol, followed by a lifetime of medical management for the treatment’s side effects. That moment I realized how deeply pharmaceutical companies were tied to the very guidelines directing my care, it didn’t spark the decision. It confirmed it—especially after seeing how the initial antibiotics and steroids had already started turning things around.
A Rare Diagnosis—and Rare Consent
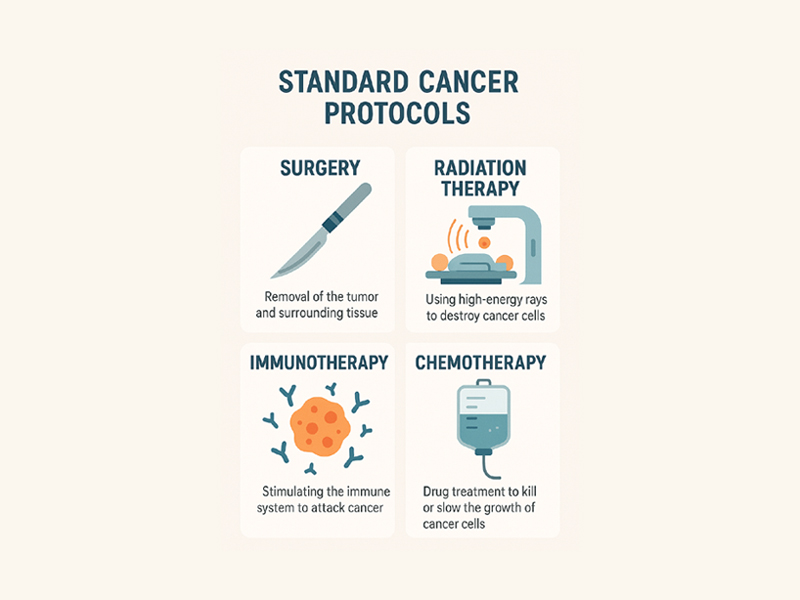
My cancer—Primary Central Nervous System Lymphoma (PCNSL)—is considered rare. That label triggered a specific sequence of events: a diagnosis fast-tracked into treatment, multiple high-risk drugs, and a deeply standardized regimen. What I didn’t fully understand at the time was that many of the drugs in this protocol were either off-label or experimental.
- Temozolomide depletes lymphocytes—the very cells I needed to heal.
- I had severe reactions to Rituximab (violent immune response).
- Vancomycin (severe allergic reaction).
The NCCN guidelines for my disease recommended 12 different clinical trials for the “let’s beat cancer team” and had one brief phrase about healthy living. At no point did anyone stop to ask why I had this cancer—what my body had been exposed to, whether my long-standing dental infection, systemic inflammation, or chemical sensitivities played a role. Instead, I was swept into the treatment protocol: no pause, no curiosity, and no exploration of root causes.
The Orphan Drug and Rare Disease Loophole
My diagnosis—Primary Central Nervous System Lymphoma (PCNSL)—qualifies as a rare disease under the Orphan Drug Act of 1983 and the Rare Diseases Act of 2002, laws enacted to spur research and development for conditions affecting fewer than 200,000 Americans (the threshold for ‘rare’ under the ODA). The intention was noble. The outcome? More slippery. Drugs approved under these acts often:
- Benefit from flexible FDA approval standards, like smaller trials or surrogate endpoints.
- Receive tax credits of up to 25% for clinical trial costs.
- Access competitive federal grants from FDA and NIH, ranging from hundreds of thousands to millions.
- Waive FDA user fees, saving companies millions per application.
- Gain seven years of market exclusivity for the orphan use—even for off-patent drugs.
In practice, this allows companies to profit from drugs integrated into rare disease protocols, without necessarily demonstrating improved outcomes. And since guidelines like NCCN’s are developed by experts at institutions receiving funding from those same companies, the incentives form a tight circle. According to their own 2020–2023 patient guidelines, the NCCN’s recommendations are based largely on expert opinion and trial availability—not robust long-term outcomes. Even the most recent protocols continue to suggest clinical trials and immunochemotherapy combinations, while failing to acknowledge root causes or preventative strategies. I wasn’t just a patient. I was a participant in a commercial enterprise.
The Economics of “Standard of Care”
Date of Service | Procedure Description | DX Code | Qty | Charge | Price per Unit | Brand |
12/7/23 | Rituximab-PVR Injection | G93.89 | 10 | $1,909.20 | 190.92 | PFIZER BRAND NAME |
12/7/23 | Rituximab-PVR Injection | G93.89 | 50 | $9,545.80 | 190.92 | PFIZER BRAND NAME |
12/21/23 | Rituximab-ABBS Injection | C85.89 | 10 | $2,343.90 | 234.39 | BIOSIMILAR DRUG |
12/21/23 | Rituximab-ABBS Injection | C85.89 | 50 | $11,719.40 | 234.39 | BIOSIMILAR DRUG |
One look at my Mayo Clinic itemized bill told the other half of the story. The pharmaceutical charges were staggering and volatile fluctuating dramatically in a short time frame:
A baffling increase for a “Bio-Similar” drug in such a short time, possibly due to quarterly adjustments in average sales price or hospital markups.
Adding insult to injury, insurance denied coverage in the end because Rituximab is typically for B-cell lymphomas (it targets CD20 on B-cells), and mine did not have involved B-cells.
But it wasn’t just the cost. It was the pattern.
These were the exact drugs I reacted poorly to—triggering violent immune responses, allergic reactions, or systemic crashes. And the manufacturers? Genentech for Rituximab. Pfizer for Vancomycin. Merck and generic suppliers for Temozolomide. Ironically—or perhaps not—Genentech and Pfizer were among the names that briefly appeared at the top of the NCCN guidelines page that night.
These weren’t just medications. They were products in a pipeline—and I was part of the distribution system.
- Legal.
- All of it codified.
- None of it is transparent.
The 2022 NCCN Form 990 shows over $23 million in revenue, with “educational grants” from pharmaceutical firms and corporate “supporters” often tied to the drugs featured in their guidelines. Their own foundation, which funds emerging cancer researchers, lists Genentech, Karyopharm Therapeutics, and Servier as major supporters. The incentives are clear. But for the patient, it’s a blur of white coats and urgency—impossible to distinguish scientific rigor from sales strategy.
What I Did Instead
In January 2024, I made the choice to step off the conveyor belt. It wasn’t out of fear. It was out of awareness.
I turned toward a different path: one grounded in whole-body healing and immune system restoration.
As I reflect on the journey, I realize now that the massive antibiotic regimen combined with steroids I received from the Mayo Clinic the first week I was there put a stranglehold on the infection that was ravaging my lymphatic system, but it did not totally eliminate the infection that was still brewing.
By January 4, 2024, my MRI already showed a positive response to this initial treatment—with a significantly decreased zone of T2 signal abnormality and enhancement in the biopsy-proven CNS lymphoma centered on the posterior body of the corpus callosum, plus no new or progressive enhancement—proving the infection was being addressed without the full chemo protocol they were pushing so urgently.
In addition to dental work (i.e., the removal of the problematic tooth), healing came and continues with holistic practices such as:
- Breathwork.
- Initially, an extended fast to trigger neural autophagy.
- Clean nutrition and mitochondrial support.
- Daily detoxification through sauna and exercise.
- Elimination of all endocrine-disrupting chemicals in my consumed products.
These elements of care help illustrate what is often missing from Standard Cancer Protocols: patient-centered approaches.
Was it scary to walk away from a world-renowned protocol? Yes. But what was scarier was not asking questions.
What the Data Says Now
My last scan, a June 2025 MRI, showed “No masses. No new white matter abnormalities. No active disease.” I expect my next scan (and what will be my last scan; I am done with the waiting and watching for the other shoe to drop) will be stellar.
- I’m not claiming that breathwork cured me.
- I’m not suggesting that fasting is a magic bullet.
But I am saying this: The system I walked away from didn’t acknowledge what I now believe was driving my illness. And the healing I’ve experienced didn’t come from the protocol. It came from reclaiming agency.
Why I’m Telling You This
Because I’m not alone.
Thousands of patients are being routed into systems that serve drug development, not true recovery.
If you are facing a diagnosis—especially a rare one—ask who benefits. Ask whether your protocol is backed by long-term remission data or short-term pharmaceutical incentives.
Ask why root causes are rarely explored.
- You are not a diagnosis.
- You are not a data point.
- You are not their bottom line.

Linda Wulf
Linda Wulf is a cancer rebel, advocate, and independent researcher. Diagnosed in 2023 with primary CNS lymphoma, she declined standard chemotherapy and pursued a root-cause, immune-supporting path. Twenty-three months cancer-free via root-cause approach.